Products > TruDiagnosis® > TruArray Warfarin Assay
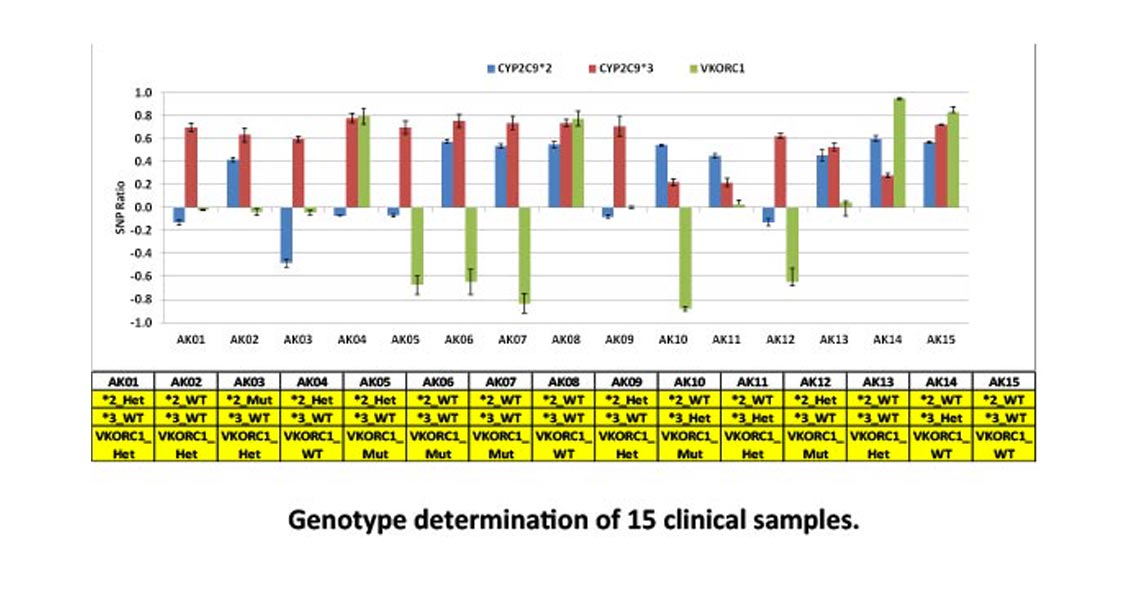
A qualitative test for the detection and genotyping of the single nucleotide polymorphisms (SNPs) in CYP2C9 gene of the cytochrome P450 enzyme, CYP2C9*2 (C430T), CYP2C9*3 (A1075C), and vitamin K epoxide reductase C1 (VKORC1) gene promoter -1639 (or 3673) G>A, as an aid in the identification of increased warfarin sensitivity.
The TruArray Warfarin Assay occurs in a single-step, closed-amplicon integrated microfluidic microarray consumable (IMMC) for a simplified microarray detection approach. This system combines multiple steps into a simple, compact workflow without the need for complex instrumentation or consumables, and is less susceptible to contamination than conventional methods.
The assay has been tested extensively with saliva samples and shown to be robust with reliable and highly accurate results. The simplified workflow can be performed in less than 3 hours for a sample-to-answer result.
* All of Akonni Biosystems tests are currently for Research Use Only, and are not for use in diagnostic procedures.
© 2003-2025 Akonni Biosystems, Inc. All rights reserved. Terms and Conditions • Privacy Policy
TruDiagnosis Systems, TruDx Readers, TruArray Tests, TruTip Kits, Primers, Lysis and Binding Buffers, Wash Buffers and Elution Buffers: For Research Use Only. Not for use in diagnostic procedures. No claim or representation is intended to provide information for the diagnosis, prevention or treatment of a disease.

Based on its recent analysis of the microarrays in molecular diagnostics (MDx) market, Frost & Sullivan recognizes Akonni Biosystems with the 2017 North American New Product Innovation Award.
Akonni’s integrated MDx system, TruDiagnosis®, is revolutionizing the point-of-care molecular testing market by enabling target detection from a variety of sample types. TruDiagnosis® is powered by TruArray®, a patented 3D gel-drop microarray technology for sample screening that instantly indicates the presence of disease markers in real time.
Download: